Since the introduction of the drugs Nivolumab and Pembrolizumab in 2014, PD-1/PD-L1 immune checkpoint inhibitors (CHIs) have turned into one of the most powerful therapeutic strategies against many types of cancer. These “passive” antibody therapies aim at enhancing the body’s own immune response against cancer, rather than specifically targeting tumor cells. Thus, the outcome of therapeutic strategies using PD-1/PD-L1 inhibitors is highly dependent on both the systemic immune status of a patient as well as on the specific tumor microenvironment. Perhaps not surprisingly, in some patients PD-1/PD-L1 inhibitors are remarkably effective against advanced tumors that were deemed untreatable only a few years ago while, in other patients, the same treatment shows no effect whatsoever.
The clinical success of PD-1/PD-L1 immune checkpoint inhibitors has led to a surge of studies to find ways to identify patient populations that respond well to these treatments and to enhance the clinical outcome of these new drugs for selected patient populations. Thus, numerous studies are attempting to identify biomarkers that can be used to predict the outcome of PD-1/PD-L1 immune checkpoint inhibitors in specific therapeutic indications for individual patients and there is a growing list of biomarkers investigated for each cancer type. More importantly, over the past few years, the effects of PD-1/PD-L1 inhibitors either alone or, increasingly, in combination with other therapeutic approaches, have been studied in thousands of clinical trials worldwide [1]. Many of these ongoing clinical studies are focused on therapeutic approaches or tools that are available to clinicians today and most are largely “experimental” in nature—in other words, most of these studies investigate the empirical effects of numerous treatment variations or treatment combinations while treating a patient’s immune system essentially as a “black box”.
In a remarkable new paper published in Nature by Sidonia Fagarasan, Professor for Integrated High-Order Regulatory Systems at the Center for Cancer Immunotherapy and Immunobiology (CCII) and Team Leader in the Laboratory for Mucosal Immunity at the RIKEN Center for Integrative Medical Sciences in Yokohama, and her collaborators have taken a very different approach that goes back to the very basics of how the immune system responds to challenges. The behavior of immune cells is thought to be largely determined through “signals” that originate from complex molecules, such as soluble or cell-bound proteins. But, immune tissues also produce and consume a significant number of small, water-soluble metabolites, which is not surprising, especially given that any immune response is a highly dynamic, energetically demanding process. Thus, Fagarasan and her colleagues hypothesized that small, water-soluble metabolites found in various immune tissues may also influence the behavior of immune cells and, thus, may exhibit “signaling” potential.
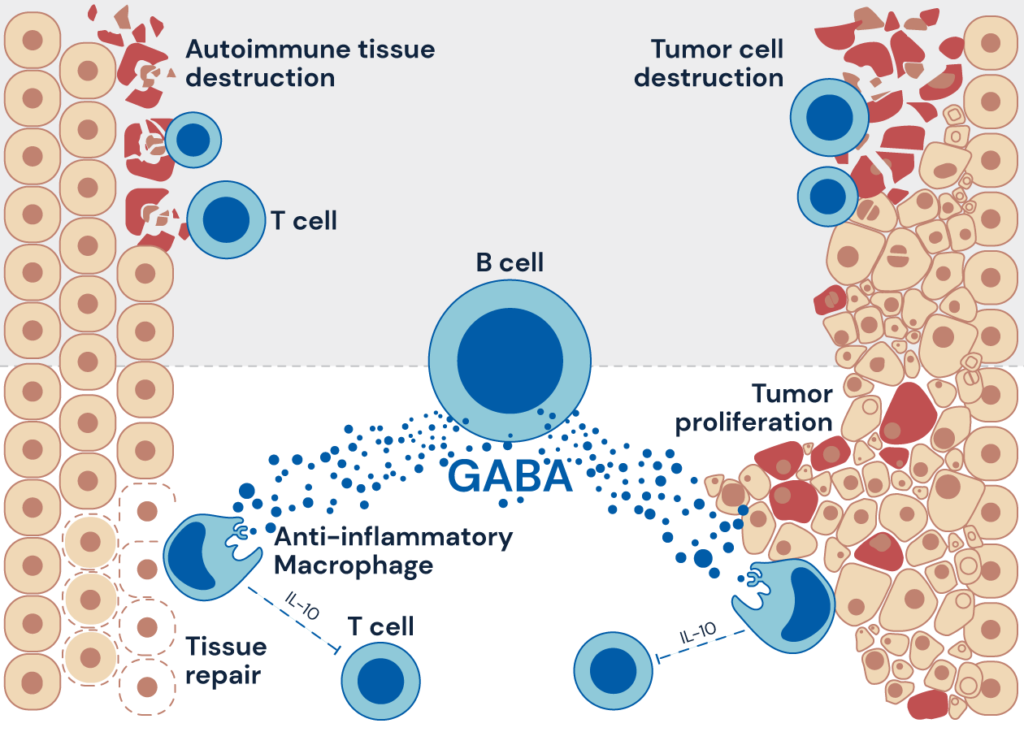
Figure: B cell-derived GABA elicits IL-10+macrophages to limit anti-tumour immunity
Source: CCII.
To investigate this hypothesis, the authors started off with a simple but ingenious use of a classical mouse foot-pad immunization model, and then investigated metabolites in lymph nodes near the immunization side versus resting lymph nodes on the opposite, non-immunized foot using an impressive arsenal of state-of-the-art technologies in metabolomic, proteomic, and genetic analysis. Around 200 metabolites were identified showing a significant difference in abundance in the two types of lymph nodes, with most of the difference shown in the alanine, aspartate and glutamine pathways. Further studies in mice deficient in major types of lymphocytes confirmed immune activation as the main factor in the metabolomic shift between the two types of lymph nodes and further revealed a surprising finding: the neurotransmitter GABA (Gamma-aminobutyric acid) was shown to be the main metabolite present in the stimulated lymph nodes, produced almost exclusively by B-cells, the type of immune cells that produces antibodies to fight bacteria and viruses.
Next, the authors looked at a common cancer model in which B-cells are known to inhibit anti-tumor activity. While B-cell deficient mice were able to control tumor growth better, the implant of pellets that slowly release GABA resulted in an increase in tumor growth and reduced activity of T-cell, the types of lymphocytes that can attack cancer cells more directly. By contrast, mice lacking the ability to produce GABA in B-cells exhibited delayed tumor growth and enhanced T cell activity. Further, the authors also found that GABA facilitates the differentiation, proliferation and survival of anti-inflammatory macrophages, immune cells that play a critical role in the initiation, maintenance, and resolution of inflammation.
Most of the results for this paper were derived from studies with mouse models. When, after the initial submission to the journal Nature, one reviewer challenged the authors and asked whether human B-cells can also convert glutamine to GABA, the authors then added a series of experiments tissues derived from human donors, including samples for rheumatoid arthritis and renal cancer patients provided by the Kyoto University Hospital. Remarkably, Fagarasan and her colleagues found that human B-cells are equally capable of producing GABA, which may explain why the presence of B-cells in certain cancers is correlated with immune evasion and poor prognosis.
These surprising new findings have broader implications, both for fundamental research in immunology and systems biology and for immunotherapy strategies in infectious diseases, autoimmune diseases, and cancer. GABA was first isolated in plants where it was shown to be produced in response to environmental stress while in the mammalian central nervous system GABA’s principal role is to reduce neuronal excitability. In autoimmune disease and inflammation, the presence of GABA may well play a beneficial role. But, in tumors, GABA produced by B-cells causes an increase in anti-inflammatory macrophages which, in turn, lead to a reduction of the anti-tumor activity by T-cells.
More generally, this surprising new paper demonstrates that water-soluble small metabolites do play a significant role in orchestrating the complex interactions between various types of immune cells. While the relevance of these findings goes well beyond cancer immunotherapy, this paper demonstrates that enhancing the outcome of existing cancer immunotherapy treatments will require significant investments in fundamental research aimed at a better understanding of the immune response in various types of human cancers.
[1] 1 A survey by the Cancer Research Institute identified a total of 4400 clinical studies involving PD-1/PD-L1. See: Samik Upadhaya et al., “Combinations take centre stage in PD1/PDL1 inhibitor clinical trials”, Nat. Rev. Drug Discov. 20, 168–169; 2021. Accessed at: https://www.nature.com/articles/d41573-020-00204-y.
Reference
Zhang, B., Vogelzang, A., Miyajima, M. et al. B cell-derived GABA elicits IL-10+macrophages to limit anti-tumour immunity. Nature 599, 471–476 (2021). DOI: https://doi.org/10.1038/s41586-021-04082-1
Contact
Professor Sidonia Fagarasan, Division of Integrated High-Order Regulatory Systems
