Members
Professors

Prof. Tasuku Honjo

Nasim Begum

Maki Kobayashi

Kenji Chamoto

Background
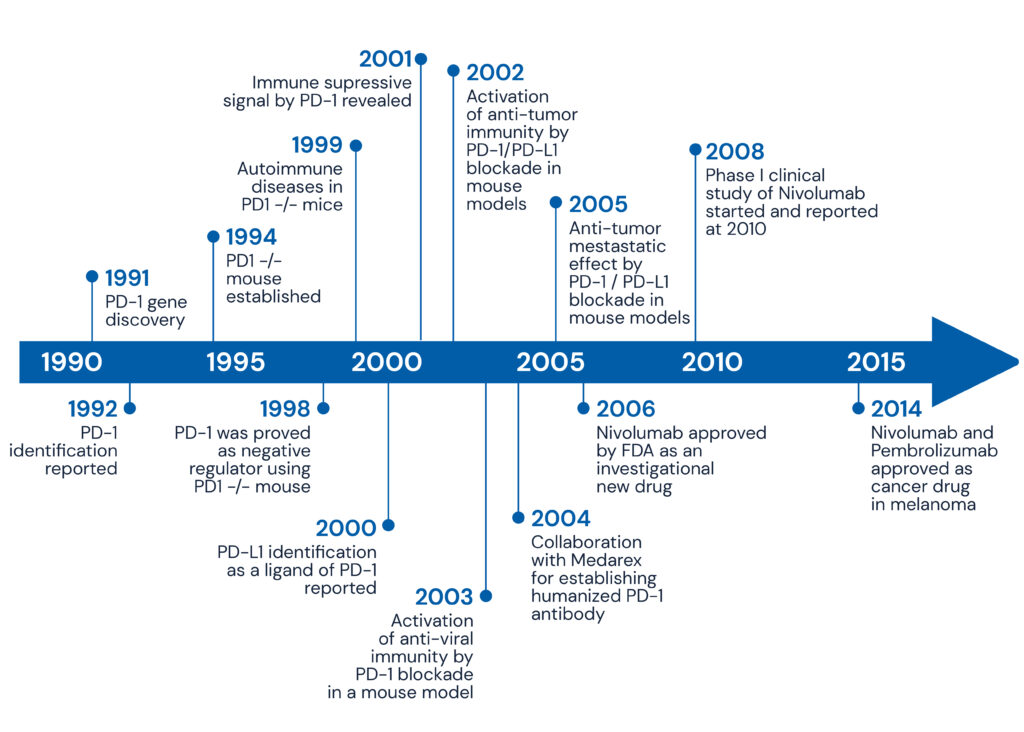
PD-1 (“Programmed cell Death 1”) was isolated and identified by Dr. Ishida and his colleagues in 1992 as a gene whose expression is upregulated during T cell death induction. In 1998, PD-1-deficient mice developed splenomegaly, with increased blood immunoglobulin levels, and increased responsiveness of splenic B cells to anti-IgM stimulation, indicating that PD-1 negatively regulates immune responses in vivo. The autoimmune disease caused by PD-1-deficient mice differs depending on the strain of mice, with the C57BL/6 strain developing SLE-like nephritis and arthritis, and the BALB/c strain developing dilated cardiomyopathy. Using these models, we are analyzing the pathogenesis of autoimmune diseases caused by PD-1, and we are developing methods to control immune responses and treat autoimmune diseases by regulating PD-1 signaling.
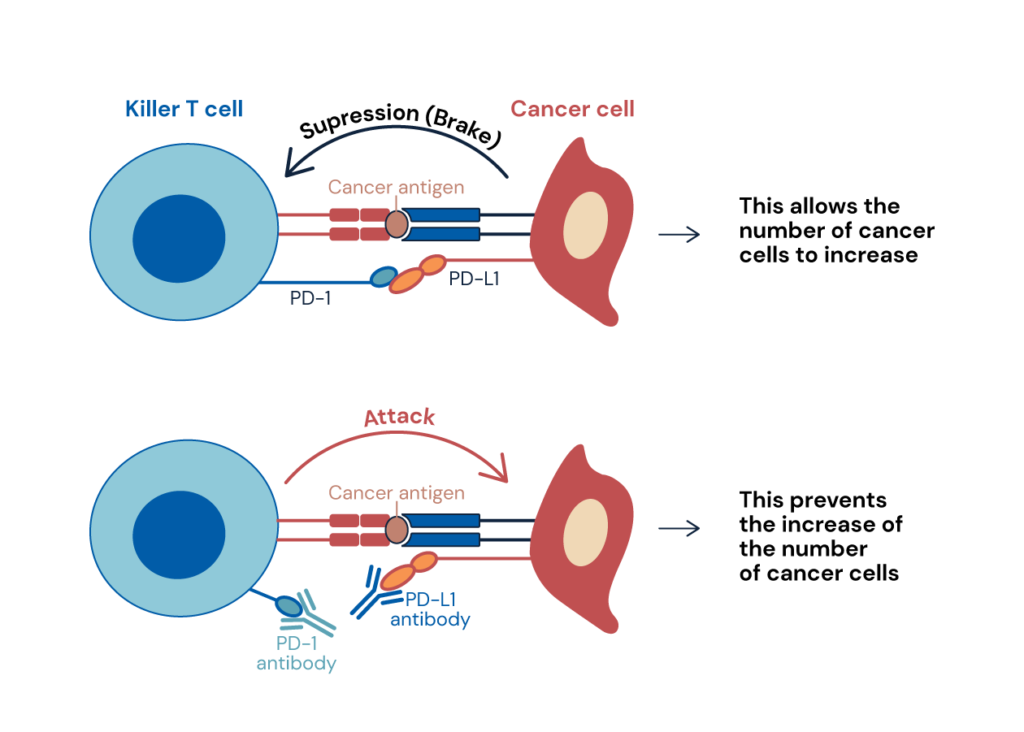
Moreover, we are studying tumor immunity which is closely related to autoimmunity. Tumors have traditionally been regarded as part of the autologous tissue. Therefore, it is thought that immunity cannot reject tumors because it cannot distinguish between self and non-self. However, the fact that PD-1-deficient mice develop autoimmunity indicated that blocking PD-1 signaling may allow them to attack tumors. Based on this hypothesis, we have shown that tumor cells express PD-L1 and escape immune surveillance. In humans, the expression of PD-L1 was higher in cancer patients with poor prognosis than in those with good prognosis, and administration of PD-1 or PD-L1 antibodies to tumor-bearing mice led to the release of inhibitory signals and activation of T cells, which suppressed tumor growth and metastasis (see Figure 1).
Figure 1: Immunotherapy through Antibody Blockade of the PD-1/PD-L1 Immune Checkpoint Pathway
Based on these results, our laboratory was the first in the world to advocate PD-1 antibody-based cancer immunotherapy, and clinical trials in cancer immunity with nivolumab (Opdivo) began in 2006. In July 2014, PD-1 antibody was approved for the treatment of malignant melanoma for the first time in Japan and abroad, and clinical trials using PD-1 antibody are now being conducted around the world.
Current Research
Research on PD-1
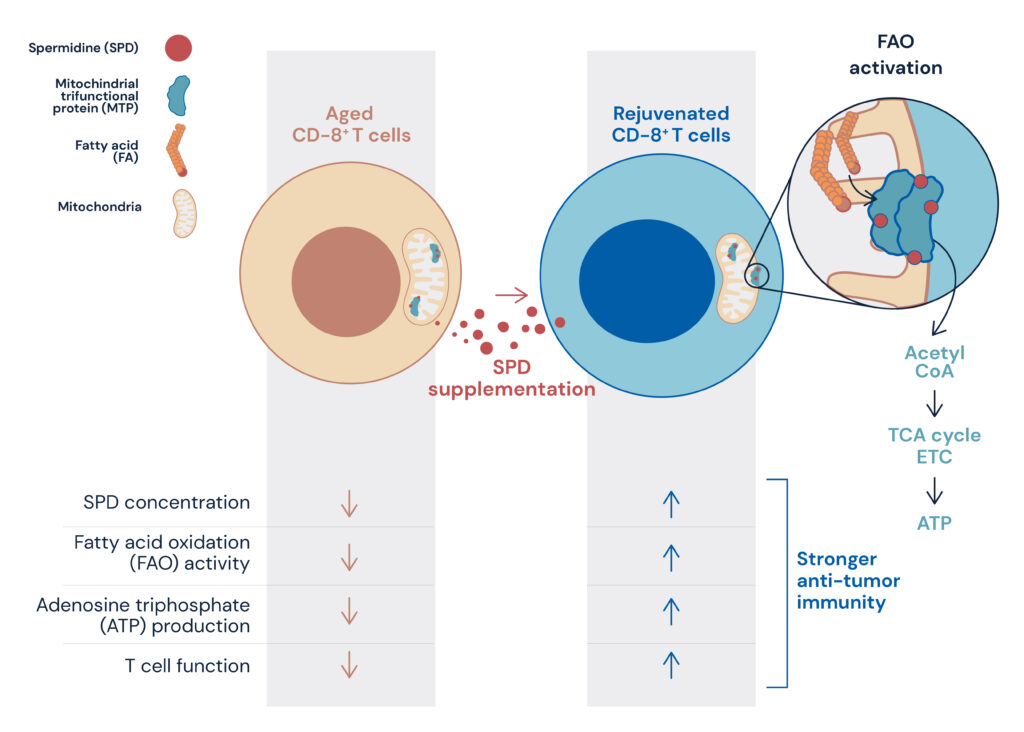
More recently, it has been discovered that PD-1-mediated immune responses are intricately connected to aging, metabolism, and stress responses, playing a crucial role in maintaining essential homeostasis for sustaining life. Notably, studies have highlighted the significance of mitochondrial metabolism in supporting T-cell functionality during PD-1 inhibitory antibody treatment. In a mouse model, substances such as bezafibrate and spermidine, known to enhance mitochondrial metabolism, specifically fatty acid oxidation, have shown to augment the therapeutic efficacy of PD-1 inhibitory antibody treatment by bolstering T-cell function (see figure 1).
In collaboration with the Department of Respiratory Medicine at Kyushu University School of Medicine, a Phase I clinical trial investigating the combination of bezafibrate with PD-1 antibodies was conducted. The trial demonstrated that this combination therapy could enhance progression-free survival (PFS) in patients with EGFR mutation-negative lung cancer.
Furthermore, the role of spermidine in mitochondrial metabolism has been highlighted, as it directly interacts with enzymes involved in fatty acid oxidation. During the aging process, T cells experience reduced levels of spermidine, leading to impaired mitochondrial function. However, spermidine supplementation has shown the potential to restore mitochondrial function in T cells (AI-Habsi et al. Science, 2022).
Moving forward, our research will further explore the integration of immunity, metabolism, and stress responses, aiming to analyze the underlying principles of biological phenomena from this innovative perspective. These insights will be crucial in advancing cancer immunotherapy, as highlighted in a recent publication.
Our goal is to foster independent researchers who are equipped with the ability to view life as a whole by learning molecular biological and immunological experimental techniques and the philosophy of living organisms. We are always looking for highly motivated students and post-doctoral fellows who are willing to work at the forefront of the world and tackle fundamental questions of immune phenomena.
Research on Activation-Induced cytidine Deaminase (AID): Unveiling the Secrets of Genomic Diversity
Over almost four decades, this group has made numerous contributions to our understanding of the antibody diversification processes. B lymphocytes are special immune cells with internal mechanisms to reconfigure their own genome. It is these gene alterations that allow B cells to generate a sheer unlimited diversity of immunoglobulins. This, in turn, enables B cells to recognize and counteract a very wide range of pathogens.
Over the past few decades class switch recombination (CSR) and somatic hypermutation (SHM) have been identified as fundamental genetic processes at the center of generating antibody diversity. Already in the 1970s and 1980s our group has made various contributions to the understanding of the molecular mechanism of class switch recombination (CSR), including the “deletion model” suggested in 1978, the identification of “S regions” as targets of CSR in 1980, and the cloning of IL-4 and IL-5, both of which are crucial in CSR in 1986.
In 1999, we identified and cloned Activation-Induced Cytidine Deaminase (AID), an enzyme specifically expressed in in germinal centers of lymphoid organs in B lymphocytes, the site of class switching and somatic hypermutation (SHM). As the name implies, AID becomes active only upon antigen activation of mature B cells and induces DNA cleavage, essential for the genetic alteration processes induced by class switch recombination (CSR) and somatic hypermutation (SHM).
How a relatively small cytidine deaminase like AID can precisely target megabase-sized molecules such as IgH has long been a mystery. The unraveling of AID’s function and mechanism of action has introduced a series of DNA break and repair modulators and facilitators, ranging from chromatin remodeling to RNA-binding proteins. AID-induced recombination is intricately intertwined with transcription, splicing, structured RNA/DNA, and the involvement of regulatory non-coding RNA, creating a complex web of interactions. How AID is involved in both DNA cleavage and recombination remains a topic for further investigation.
We have shown that AID induces class switching and hypermutation not only in B cells but also in non-B cells, establishing its role in genetic alterations driven by antigen stimulation. AID is both necessary and sufficient for these alterations in response to antigen stimulation in mammalian B cells, establishing AID as a prominent mutator in vertebrates.
Unwanted genome rearrangements caused by AID are also involved in various malignancies, including B-cell lymphomas, autoimmune disorders, and cancer. AID and members of the APOBEC protein family are increasingly investigated as a target for potential therapeutic intervention in autoimmune diseases and cancer. Further, leveraging the power of C/dC-deamination, the AID/APOBEC protein family has suggested new approaches for direct base editing of the genome, a forward-looking technology that extends well beyond B cells.
We continue to investigate the complex role of AID in genetic diversity and, more generally, the processes that allow multicellular organisms to generate genetic diverse and elaborate biological systems from a limited gene pool. While considerable progress has been made over the past two decades, research on AID remains a relatively young field and many questions remain to be answered. Whatever the answers will be, the many intricacies of AID will likely provide us with a deeper comprehension of both immunity and genetic evolution, with implications that extend far beyond the realm of basic biology.
Selected Publications
PD-1
Hayashi H, Chamoto K (Co-corresponding author), Hatae R, Kurosaki T, Togashi Y, Fukuoka K, Goto M, Chiba Y, Tomida S, Ota T, Haratani K, Takahama T, Tanizaki J, Yoshida T, Iwasa T, Tanaka K, Takeda M, Hirano T, Yoshida H, Ozasa H, Sakamori Y, Sakai K, Higuchi K, Uga H, Suminaka C, Hirai T, Nishio K, Nakagawa K, Honjo T. Soluble immune checkpoint factors reflect exhaustion of antitumor immunity and response to PD-1 blockade. (2024) J Clin Invest. 134(7):e168318. DOI: https://doi.org/10.1172/JCI168318
Chamoto, K., Yaguchi, T., Tajima, M., Honjo, T. Insights from a 30-year journey: function, regulation and therapeutic modulation of PD1. Nat Rev Immunol. 2023 Apr 25. DOI: 10.1038/s41577-023-00867-9.
Al-Habsi, M., Chamoto, K., Matsumoto, K., Nomura, N., Zhang, B., Sugiura, Y., Sonomura, K., Maharani A., Nakajima Y., Wu, Y., Nomura, Y., Menzies, R., Tajima, M., Kitaoka, K., Haku, Y., Delghandi, S., Yurimoto, K., Matsuda, F., Iwata, S., Ogura, T., Fagarasan, S., Honjo, T. Spermidine activates mitochondrial trifunctional protein and improves antitumor immunity in mice. Science. 28; 378 (6618): eabj3510. (2022). DOI: 10.1126/science.abj3510
Iwai, Y., Ishida, M., Tanaka, Y., Okazaki, T., Honjo, T., Minato, N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. PNAS 99, 12293-7 (2002). DOI: https://doi.org/10.1073/pnas.192461099
Nishimura, H., Okazaki, T., Tanaka, Y., Nakatani, K., Hara, M., Matsumori, A., Sasayama, S., Mizoguchi, A., Hiai, H., Minato, N., Honjo, T. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001 Jan 12;291(5502):319-22. DOI: 10.1126/science.291.5502.319
Ishida, Y., Agata, Y., Shibahara, K., Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992 Nov;11(11):3887-95. DOI: 10.1002/j.1460-2075.1992.tb05481.x
AID
Kobayashi, M., Wakaguri, H., Shimizu, M., Higasa, K., Matsuda, F., Honjo, T. Ago2 and a miRNA reduce Topoisomerase 1 for enhancing DNA cleavage in antibody diversification by activation-induced cytidine deaminase. Proc Natl Acad Sci U S A. 2023 May 2;120(18):e2216918120. DOI: 10.1073/pnas.2216918120.
Stanlie, A., Aida, M., Muramatsu, M., Honjo, T., Begum, N.A. Histone3 lysine4 trimethylation regulated by the facilitates chromatin transcription complex is critical for DNA cleavage in class switch recombination. Proc Natl Acad Sci U S A. 2010 Dec 21;107(51):22190-5. DOI: 10.1073/pnas.1016923108.
Begum, N.A., Kinoshita, K., Kakazu, N., Muramatsu, M., Nagaoka, H., Shinkura, R., Biniszkiewicz, D., Boyer, L.A., Jaenisch, R., Honjo, T. Uracil DNA glycosylase activity is dispensable for immunoglobulin class switch. Science. 2004 Aug 20;305(5687):1160-3. DOI: 10.1126/science.1098444.
Muramatsu, M., Kinoshita, K., Fagarasan, S., Yamada, S., Shinkai, Y., Honjo, T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000 Sep 1;102(5):553-63. DOI: 10.1016/s0092-8674(00)00078-7.
Honjo, T., Kataoka, T. Organization of immunoglobulin heavy chain genes and allelic deletion model. Proc Natl Acad Sci U S A. 1978 May;75(5):2140-4. DOI: 10.1073/pnas.75.5.2140.
List of all publications
Recruitment & Contact
We are permanently recruiting highly motivated students, postdoctoral fellows, and technical staff regardless of previous scientific backgrounds.
Please contact us using CCII’s contact form or by directly sending an email to recruit_ccii@mail2.adm.kyoto-u.ac.jp. Please indicate in which position and subject area you are interested in and that your inquiry is about our research department.