Mission
Development of next-generation cancer immunotherapies that enhance the efficacy of PD-1 blockade.
Members
Associate Professor



Research Overview
Our division was established in April 2023. The great clinical achievements of checkpoint inhibitors (CPIs) have demonstrated that the immune system is capable of eradicating tumor cells, validating the concept of harnessing the patient’s own immune system to control cancer. However, only a minority of patients respond to CPIs, highlighting the unmet medical need for new immunotherapies.
Cancer immunity is triggered at the tumor site by the innate immune activation and the antigen release followed by its uptake by dendritic cells. In situ vaccines, which artificially “activate innate immunity” and “promote the release of cancer antigens” at the tumor site, induce cancer immunity mostly upstream. Therefore they are expected to synergize with any other classes of cancer immunotherapies including CPIs. In addition, they can (1) induce systemic and long-lasting memory responses in the setting of localized treatment with minimized adverse effects and (2) utilize patient-specific cancer antigens as vaccine antigens, thereby achieving personalized vaccination without the need for complicated processes, such as antigen isolation and identification, which are required for neoantigen vaccine strategy. Thanks to recent advances in medical devices such as endoscopy, endoscopic ultrasound, ultrasound, and intra-arterial catheter, therapeutic intervention can be easily achieved, facilitating clinical application of in situ vaccines. Importantly, because these modalities are universally applied in various medical fields, the in situ vaccine strategy can be applied to various cancer types.
Figure: Enhanced innate immune activation and antigen release at the tumor site leverage PD-1 blockade
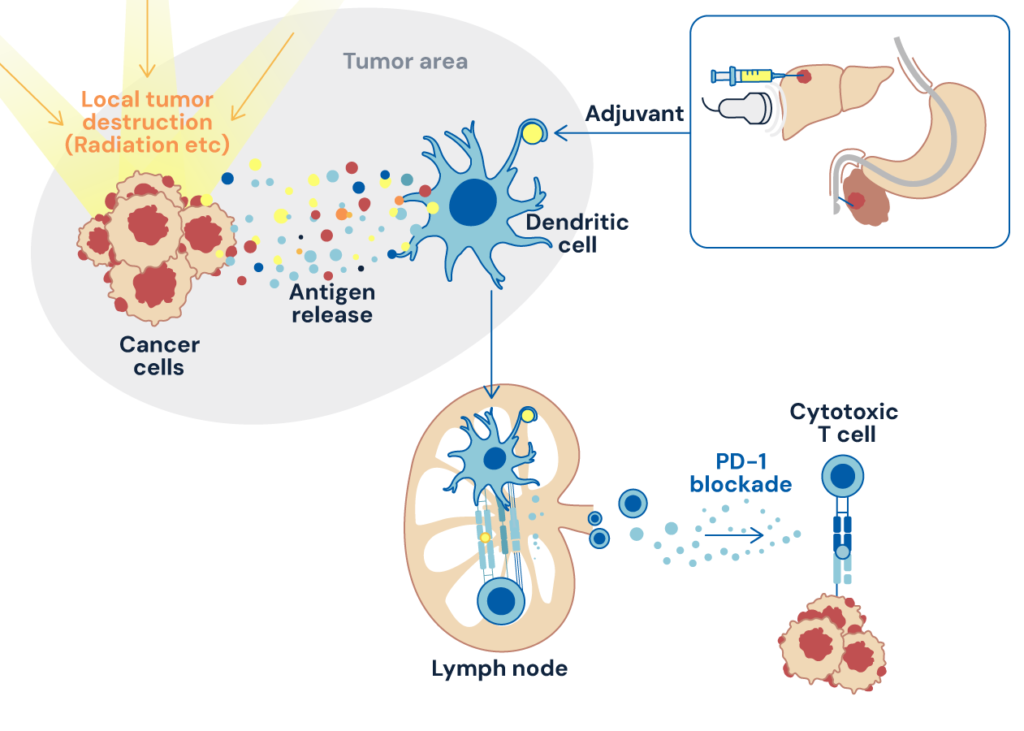
The primary goal of our division is to develop in situ vaccines that maximize cancer immune activation by immunological intervention at tumor sites using a two-sided approach, i.e., “unique innate immunity-activating adjuvant” and “enhanced tumor destruction to release patient’s own tumor antigens”. This strategy, applied in therapeutic and neoadjuvant settings, will synergize with PD-1 blockade, leading to cutting-edge cancer immunotherapy.
Selected Publications
Yaku, H., Takahashi, K., Okada, H., Kobiyama, K., Shiokawa, M., Uza, N., Kodama, Y., Ishii, K.J., Seno, H. Near-infrared photoimmunotherapy as a complementary modality to in situ vaccine in a preclinical pancreatic cancer model. Biochem. Biophys. Res. Commun. 737, 150534, 2024. DOI:10.1016/j.bbrc.2024.150534
Okada, H., Takahashi, K., Yaku, H., Kobiyama, K., Iwaisako, K., Zhao, X., Shiokawa, M., Uza, N., Kodama, Y., Ken J, Ishii., Seno, H. In situ vaccination using unique TLR9 ligand K3-SPG induces long-lasting systemic immune response and synergizes with systemic and local immunotherapy. Sci Rep 12, 2132 (2022). DOI: 10.1038/s41598-022-05702-0.
Eso, Y., Takeda, H., Taura, K., Takai, A., Takahashi, K., Seno, H.. Pretreatment Neutrophil-to-Lymphocyte Ratio as a Predictive Marker of Response to Atezolizumab Plus Bevacizumab for Hepatocellular Carcinoma. Curr Oncol 28, 4157-4166, 2022. DOI: 10.3390/curroncol28050352.
Nakano, S., Eso, Y., Okada, H., Takai, A., Takahashi, K., Seno, H.. Recent Advances in Immunotherapy for Hepatocellular Carcinoma. Cancers (Basel) 12, 2020. DOI: 10.3390/cancers12040775
Yoshiji, S., Takahashi, K., Sawada, K., Mishima, M., Eso, Y., Morita, M., Takai, A., Marusawa, H., Ueda, Y., Mimori, T., Seno, H.. Accelerated Progression of Hepatocellular Carcinoma during Immunosuppressive Therapy with Abatacept for Rheumatoid Arthritis. Intern Med 58, 67-71, 2019. DOI: 10.2169/internalmedicine.0843-18.
Wieland, SF., Takahashi, K., Boyd, B., Whitten-Bauer, C., Ngo, N., de la Torre, JC., Chisari, F.V.. Human plasmacytoid dendritic cells sense lymphocytic choriomeningitis virus-infected cells in vitro. J Virol. 88, 752-7, 2014. DOI: 10.1128/JVI.01714-13
Kumar, H., Pandey, S., Zou, J., Kumagai, Y., Takahashi, K., Akira, S., Kawai, T.. NLRC5 deficiency does not influence cytokine induction by virus & bacteria infections. J Immunol 186, 994-1000, 2011. DOI: 10.4049/jimmunol.1002094
Takahashi, K., Asabe, S., Wieland, S., Garaigorta, U., Gastaminza, P., Isogawa, M., Chisari, F.V.. Plasmacytoid dendritic cells sense hepatitis C virus-infected cells, produce interferon, & inhibit infection. Proc Natl Acad Sci U S A 107, 7431-7436, 2010. DOI: 10.1073/pnas.1002301107.
Takahashi, K., Kawai, T., Kumar, H., Sato, S., Yonehara, S., Akira, S.. Roles of caspase-8 & caspase-10 in innate immune responses to double-stranded RNA. J Immunol 176, 4520-4524, 2006. DOI: 10.4049/jimmunol.176.8.4520.
Kumar, H., Kawai, T., Kato, H., Sato, S., Takahashi, K., Coban, C., Yamamoto, M., Uematsu, S., Ishii, K.J., Takeuchi, O., Akira, S.. Essential role of IPS-1 in innate immune responses against RNA viruses. J Exp Med 203, 1795-1803, 2006. DOI: 10.1084/jem.20060792.
Ishii, K.J., Coban, C., Kato, H., Takahashi, K., Torii, Y., Takeshita, F., Ludwig, H., Sutter, G., Suzuki, K., Hemmi, H., et al.. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat Immunol 7, 40-48, 2006. DOI: 10.1038/ni1282.
Kawai, T., Takahashi, K., Sato, S., Coban, C., Kumar, H., Kato, H., Ishii, K.J., Takeuchi, O., Akira, S.. IPS-1, an adaptor triggering RIG-I- & Mda5-mediated type I interferon induction. Nat Immunol 6, 981-988, 2005. DOI: 10.1038/ni1243.
Recruitment & Contact
We are permanently recruiting highly motivated students, postdoctoral fellows, and technical staff regardless of previous scientific backgrounds.
Please contact us using CCII’s contact form or by directly sending an email to recruit_ccii@mail2.adm.kyoto-u.ac.jp. Please indicate in which position and subject area you are interested in and that your inquiry is about our research division.
